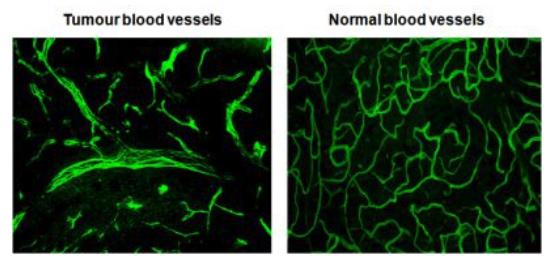
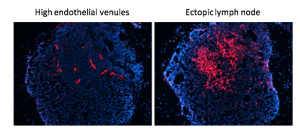
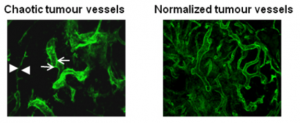
My laboratory has pioneered research into the role of the tumour microenvironment for improved anti-cancer immunotherapies with a specific focus on tumour vessel remodelling. To reach tumour cells within a cancer, immune cells must enter the tumour blood vessels, cross the vessel wall, and find their way through tumour stroma. We have shown that tortuous tumour blood vessels are a critical barrier that limits anti-cancer therapy at the tumour site. In contrast, ‘normalization’ of tumour blood vessels, the transformation of aberrant tumour vessels into a more functional or “normal” vasculature, enhances immune cell infiltration and anti-tumour immunity.
Figure 1: Blood vessels in healthy and cancerous tissue. Source: Ruth Ganss