CURRENT RESEARCH PROJECTS
Project
Project
Project Outline
• Mutations of vasopressin receptor 2 resulting in kidney disorders.
• Chemokine receptor complexes relevant to cancer and inflammatory disorders.
• Receptor tyrosine kinase complexes relevant to cancer.
• RAGE complexes relevant to cardiovascular disease.
• Orexin receptor-arrestin-ubiquitin complexes.
• Advanced resonance energy transfer technology development.
It has become clear that interactions between different receptors (known as ‘heteromerisation’) can produce quite distinct signalling and regulatory outcomes compared to activation of receptors in isolation (Mustafa et al., 2012). Therefore, understanding how receptors work in larger multi-protein complexes is critical for developing better pharmaceuticals (Gomes et al. 2016). The Laboratory for Molecular Endocrinology and Pharmacology translates its research findings through its spin-out company Dimerix (www.dimerix.com), of which Professor Pfleger is Chief Scientific Advisor. This includes the invention of the Receptor-Heteromer Investigation Technology (Receptor-HIT), which has been assigned to Dimerix from The University of Western Australia (Johnstone and Pfleger, 2012).
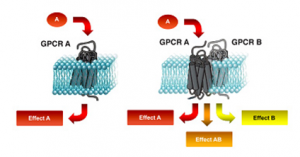
Interactions between different receptors (heteromerisation) can result in changes to receptor function (Gomes et al. 2016).
A ‘receptor heteromer’ is defined as a ‘macromolecular complex composed of at least two (functional) receptor units with biochemical properties that are demonstrably different from those of its individual components’ (Ferre et al., 2009).
Using Receptor-HIT, Professor Pfleger and colleagues have published findings demonstrating a functional interaction between angiotensin and chemokine receptors with important implications for kidney disease (Ayoub et al. 2015). This study included validation of a therapy involving inhibition of both angiotensin and chemokine receptors in pre-clinical models by colleagues at St. Vincent’s in Melbourne. Dimerix is currently undertaking Phase II clinical trials to test this therapy in patients (www.dimerix.com).
In collaboration with Professor Merlin Thomas and his team at Monash University, Professor Pfleger and laboratory colleagues have also been investigating functional interactions between angiotensin receptors and the Receptor for Advanced Glycation End-Products (RAGE), especially because of its importance in cardiovascular disease (Pickering et al., 2019).
Receptor-arrestin complexes
This laboratory has published a number of studies investigating interactions between receptors and intracellular regulatory and scaffolding molecules called β-arrestins, proteins that desensitise G protein-coupled receptors in terms of G protein signalling and facilitate their internalisation (for examples, see Kocan et al., 2009, Dalrymple et al., 2011 and Jaeger et al., 2014). There is also evidence that β-arrestins mediate signalling subsequent to G protein-mediated signalling at the plasma membrane, through the formation of a secondary signalling platform that recruits and scaffolds signalling molecules (Dromey and Pfleger, 2008). There is considerable potential for pharmaceutical development through better understanding of ‘biased signalling’, particularly with respect to plasma membrane G protein-mediated signalling versus subsequent β-arrestin-mediated signalling that may or may not involve G proteins.
Development of bioluminescence resonance energy transfer (BRET) technologies
This group are world leaders in the development and use of the bioluminescence resonance energy transfer (BRET) technology. They have published the seminal review and protocol for BRET in Nature Methods and Nature Protocols respectively. BRET utilises variants of naturally occurring proteins from marine organisms, such as the luciferase enzyme from the sea pansy, Renilla reniformis, and the green fluorescent protein from the bioluminescent jellyfish, Aequorea victoria, sometimes called the crystal jelly.
By genetically fusing the luciferase enzyme to one protein of interest such as a receptor, and fusing the fluorescent protein to another protein of interest such as β-arrestin, the proximity of these proteins can be assessed in living cells in the laboratory (Kocan and Pfleger, 2011).
BRET works because the luciferase emits blue light, but if the fluorescent protein is within 10nm, less blue light is emitted and some of the energy is transferred to the fluorescent protein through resonance – like turning forks but at the molecular level (Dacres et al., 2012). This energy is then emitted as green, yellow or orange light, depending upon the type of fluorescent protein accepting the energy transfer (Ayoub and Pfleger, 2010). As this energy transfer only occurs if the proteins are very close together, and can be detected by monitoring light emissions of specific colours, this approach can be used to provide real-time information about how the proteins are forming complexes in live cells.
This laboratory demonstrated the ability to monitor such complexes for several hours in live cells, at 37°C, in real-time, using ‘extended BRET’ (eBRET; Pfleger et al., 2006), as well as the advantages of using improved variants of Renilla luciferase and green fluorescent protein (Kocan et al., 2008).
In 2015 the laboratory, in collaboration with researchers at The University of Nottingham and Promega, demonstrated for the first time the principle of real-time ligand binding to G protein-coupled receptors (GPCRs) using BRET and published this work in Nature Methods (Stoddart et al., 2015). This provides an important alternative to radioligand binding experiments and extends researchers’ capability to allow assessment of real-time binding kinetics in live cells.
In 2016, the laboratory published findings from BRET experiments tracking receptors in multiple different compartments within live cells in real-time (Tiulpakov et al., 2016). This means that the laboratory can now monitor multiple facets of receptor function in real-time, from ligand binding, to interactions with signalling molecules like G proteins, arrestin and Grb2, to internalisation and intracellular trafficking.
More recently, work spearheaded by Dr Carl White has resulted in seminal articles using the CRISPR/Cas9 genome editing technology to insert BRET tags on endogenous proteins of interest to enable real-time investigation of protein-protein interactions and receptor trafficking (White et al., 2017), as well as ligand binding (White et al., 2019).
Recent research highlights
The laboratory has won a number of awards for its work, including Profesor Pfleger being listed in the National Health and Medical Research Council (NHMRC) Ten of the Best Research Projects 2010 and winning the 2011 Australian Museum Eureka Prize for Emerging Leader in Science. Professor Pfleger has been honoured with an NHMRC Research Excellence Award for the top ranked fellowship application in his category and has been awarded the 2016 Novartis Prize of the British Pharmacological Society.
CURRENT STUDENT PROJECTS
Student Project
Research area: Molecular Pharmacology
Student Project
Research area: Molecular Pharmacology
Project Outline
Opportunities are available for highly motivated honours, masters and PhD candidates to be part of a leading research team, in a highly supportive and productive environment. Specific projects can be tailored to individual research interests with guidance from your supervisors. Further details are available on the Perkins website and upon request from Professor Pfleger.
Contact
Professor Kevin Pfleger – [email protected]
Chief supervisor
Professor Kevin Pfleger
Other supervisor
Elizabeth Johnstone
Project suitable for
Honours, Masters, PhD
Essential qualifications
as required for the respective degree program
Start date
flexible
