CURRENT RESEARCH PROJECTS
Project
Research Project
Project
Research Project
Project Outline
Using chemical coupling and bioengineering approaches, we are now developing imaging contrast agents and therapeutics fused with these targeting peptides for in vivo applications in pre-clinical models of cancers and atherosclerosis.
Figure 1 (below): Peptide homing (green) in vivo in tumours and plaques. Source: Unpublished
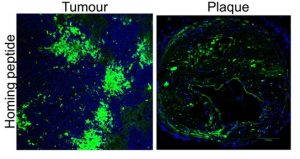
Improved diagnostic imaging for detecting cancers and atherosclerosis in vivo
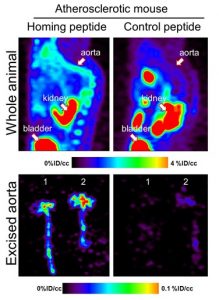
We have developed multifunctional nanoparticle-based system to carry contrast agents to image tumours and plaques using advanced imaging instrument including microPET/CT, MRI and confocal imaging. Importantly, these reagents are fused to the homing peptides to improve binding and accumulation deep in tumours and plaques.
Our goal is to be able to detect the developing tumours and atherosclerotic lesions at their earliest form as well as at the advanced chronic stages that are known to cause severe complications.
Figure 2 (below): PET imaging of atherosclerosis in vivo. Source: Hamzah et al. PNAS, 2011
Targeted therapy to destroy pathological lesions and reverse inflammation in cancers and atherosclerosis
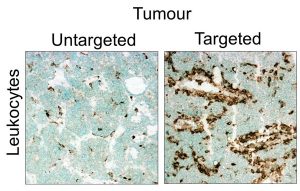
Our team explores two fundamentally different therapeutic interventions which involve targeting tumours and plaques to: i) destroy selective cellular components that contribute to the progression of cancers and atherosclerosis, and ii) re-program the diseased-promoting microenvironment by reversing specific inflammatory condition in the pathological tissues.
We engineered several classes of cell-killing agents and inflammatory mediators fused with tumour and plaque targeting peptides for effective delivery. We aim to monitor the drug penetration in tumours and plaques (by imaging) and evaluate the implications of these therapeutic strategies on cancer and plaque progression.
Figure 3 (below): Immune cell infiltration in tumours in response to targeted delivery. Source: Unpublished
Research area
Cancer, Cardiovascular Disease
Laboratory
Targeted Drug Delivery, Imaging and Therapy Laboratory
CURRENT STUDENT PROJECTS
Student Project
Designing targeted nanoparticle delivery for diagnostic imaging of cancers
Student Project
Designing targeted nanoparticle delivery for diagnostic imaging of cancers
Project Outline
This project will offer training in multidisciplinary skill sets including:
- Peptide coupling and nanoparticle production
- The use of animal models of cancers (breast carcinoma, insulinoma and hepatocellular carcinoma)
- The application advanced imaging instruments including PET, MRI and confocal microscopy for in vivo imaging of cancer
- Immunohistochemistry and biochemical assays
Contact
Assistant Professor Juliana Hamzah – [email protected]
Chief supervisor
Assistant Professor Juliana Hamzah
Other supervisor
Professor Roger Price (Nuclear Medicine, Sir Charles Gairdner Hospital), Dr Ramana Kotamraju (Sanford-Burnham Institute of Medical Research, California, USA)
Project suitable for
Honours, PhD
Essential qualifications
background in chemistry/ biochemistry
Start date
Any time.
Student Project
Developing recombinant proteins for tumour-specific delivery
Student Project
Developing recombinant proteins for tumour-specific delivery
Project Outline
Specific training provided in this project:
- Engineer immune-modulating proteins fused to our tumour-homing peptides (Technical training: cloning, protein expression, production and purification).
- Assess bioactivity of recombinant proteins (Technical training: cell culture, FACS analysis)
- Evaluate in vivo tissue homing in mouse models developing insulinoma, breast carcinoma and/or hepatocellular carcinoma (Technical training: understanding of mouse tumour models, protein labelling & imaging, histology & fluorescence microscopy).
- Evaluate short-term and long term benefits of recombinant fusion proteins in tumour-bearing mice (for PhD candidate only; technical training: Therapeutic studies, advanced immunohistochemistry and histology analysis, microscopy and advanced tissue imaging analyses).
Contact
Assistant Professor Juliana Hamzah – [email protected]
Chief supervisor
Assistant Professor Juliana Hamzah
Project suitable for
Honours, PhD
Essential qualifications
background in biology/molecular biology/immunology/pathology
