
Profile
Associate Professor Pilar Blancafort BSc, PhD completed her Bachelor of Science at the University of Barcelona, and her PhD at the University of Montreal in the field of Biochemistry in 1999. She pursued her postdoctoral studies at the Scripps Research Institute, California in the field of genome-engineering . In 2005, she established her own laboratory at the University of North Carolina, as Assistant Professor and later as tenured Associate Professor in 2011. In 2012, Professor Blancafort moved her laboratory at the University of Western Australia. In 2014, she joined the Perkins as Head of the Laboratory in Cancer Epigenetics. Pilar is a specialist in genome engineering and gene targeting and her laboratory has pioneered the development of engineered DNA binding proteins to modulate the epigenetic state of cancer cells and delivery strategies for tumor targeting in pre-clinical studies. She has received several awards, including awards from the Department of Defense Breast Cancer Program, American Lung Association, several cancer nanotechnology awards, NCI/NIH awards, a Cancer Council of Western Australia Research Fellowship and a ARC Future Fellowship and a National Breast Cancer Novel Concept award.
Research Overview
The Blancafort laboratory focuses on the development of novel approaches to target cancers that are currently refractory to treatment and associated to poor outcome, such as triple negative breast cancers and ovarian cancers. At present, there are no targeted approaches to combat these tumors with chemotherapy and radiation the only treatment options. The laboratory generates novel functionalised molecules able to specifically target these tumors with minimal toxicity to normal cells. Our emphasis is in advanced stage metastatic tumors, which quasi invariably develop resistance. Ultimately we wish to revert the behaviour of metastatic cells by sensitizing these treatment resistant tumors to chemotherapy regimes.
Recently, the genomes of thousands of cancer patients have been resolved at single base pair resolution, which has delineated the aberrant landscape of mutations, deletions and copy number amplifications that characterize the intrinsic subtypes of cancers. By integrating this information with cancer transcriptomes and DNA methylomes, a discrete number of genomic loci involved in cancer initiation and metastatic progression have been identified. Major cancer drivers include molecules such as transcription factors (e.g MYC) and small GTPases (e.g KRAS) for which there is currently no small molecule specific inhibitors. In addition, the cancer genome project has identified other elusive targets not involved in proliferative capacity, but in residual disease, drug resistance and in tumor-stromal interactions.
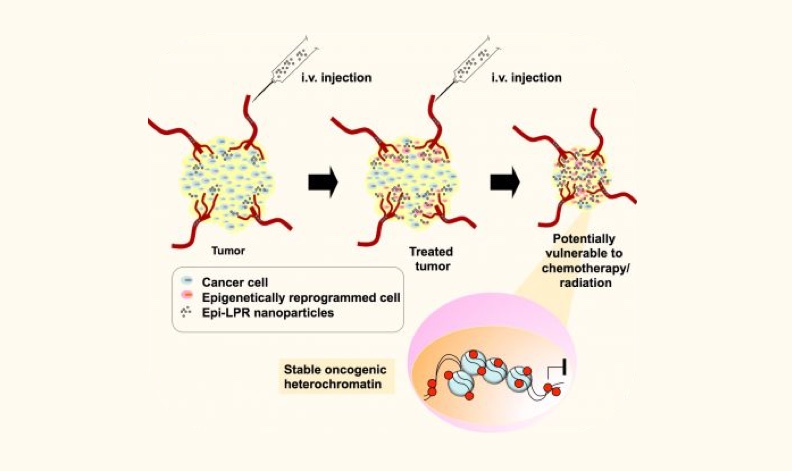
Image: Delivery of nanoparticles into tumors to promote gene silencing and sensitize cancer cells to chemotherapy
With this integrated knowledge of the cancer genome we have developed novel precision molecular medicine strategies to selectively revert or reprogram the aberrant gene expression and epigenetic state
of prospective cancer drivers in breast and ovarian tumors. We deployed state of the art molecular biology and structural biology approaches to engineer a novel generation of sequence-specific DNA-binding molecules with the capacity to recognize the cancer genome with single locus selectivity.
Structure of a transactivator like effector (TALE) DNA binding domain in complex with DNA.
These engineered DNA-binding domains (DBDs, for example made of zinc fingers, transactivator-like effector TALEs and RNA guides: CRISPR/dCas9) are designed to bind promoters, enhancers and other regulatory regions controlling the expression of the targeted loci. These DBDs are linked to effector domains able to modify and edit chromatin, for example by inducing DNA methylation or by promoting irreversible DNA damage in specific oncogenic loci. The active proteins and small peptide variants are encapsulated using nanoparticles to target the delivery of our agents in breast and ovarian cancer models. The nanoparticles incorporate both targeted ligands for tumor targeting, imaging modalities, which allow the in vivo detection of the particles using MRI and other approaches.
The outcomes of our research are the generation of novel state of the art nanoparticles for short-term clinical trials for the treatment of fatal diseases, such as metastatic ovarian cancer, and the discovery of novel therapeutic agents for these malignancies.
Structure of a transactivator like effector (TALE) DNA binding domain in complex with DNA.
Engineering of arrays of zinc finger domains binding DNA.
Research Projects
Selected Publications
Beltran A.S., Graves, L.M., Blancafort, P. Novel role of Engrailed 1 as pro-survival factor in basal-like breast cancer and engineering of interference peptides to block its oncogenic function. Oncogene. 2013 Oct 21. doi:10.1038/onc.2013.422. [NCBI PubMed Entry]
Prabhakaran, P., Hassiotou, F., Blancafort, P., Filgueira, L. Cisplatin induces differentiation of breast cancer cells. Front Oncol. (2013) Jun 3;3:134. doi: 10.3389/fonc.2013.00134. [NCBI PubMed Entry].
Hassioutou, F. Hepworth, A. Beltran, AS. Mathews, M. Stuebe, A. Hartmann, P. Filgueira, L. and Blancafort, P. Expression of the pluripotency transcription factor OCT4 in the normal and aberrant mammary gland. Front Oncol. (2013) Apr 11;3:79. doi:10.3389/fonc.2013. [NCBI PubMed Entry]
Van der Gun BTF, Huisman C., Stolzenburg S., Kazemier HG, Ruiters, MHJ., Blancafort, P. Rots MG (2013), Bidirectional modulation of endogenous EpCAM expression to unravel its function in ovarian cancer.BJC. In Press. [NCBI PubMed Entry]
Juárez-Moreno K, Erices R, Beltran AS, Stolzenburg S, Cuello-Fredes M, Owen, GI, Qian H, Blancafort P (2013). Breaking through an epigenetic wall: Re-activation of OCT4 by KRAB-containing designer zinc finger transcription factors. Epigenetics. Jan 11;8(2). [Epub ahead of print]PMID: 23314702 [NCBI PubMed Entry]
Wang Y, Su H-h, Yang Y, Zhang L, Blancafort P, Huang L. Systemic Delivery of Modified mRNA Encoding Herpes Simplex Virus 1 Thymidine Kinase for Targeted Cancer Gene Therapy. Molecular Therapy. (2012); Dec 11. doi: 10.1038/mt.2012.250. [NCBI PubMed Entry]
Blancafort P, Jin J, Frye S (2012). Writing and re-writing the epigenetic code of cancer cells: from engineered proteins to small molecules. Mol Pharmacology Minireview. Available on line http://molpharm.aspetjournals.org [NCBI PubMed Entry]
Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, Trengove N, Tat Lai C, Filgueira L, Blancafort P, Hartmann PE. Breastmilk is a Novel Source of Stem Cells with Multi-Lineage Differentiation Potential. Stem Cells. (2012) Aug 3. doi: 10.1002/stem.1188. [NCBI PubMed Entry]
Stolzenburg S, Rots MG, Beltran AS, Rivenbark AS, Yuan X, Strahl BS, Blancafort P (2012) Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Research. (2012); 40:6725-40. [NCBI PubMed Entry] Significance: This paper was selected for the Highlights of the Journal (and the cover), which comprises 5% of the papers based on innovation and scientific excellence.
Lara H, Wang Y, Beltran AS, Juarez-Moreno K, Yuan X, Kato S, Leisewitz AV, Cuello-Fredes M, Licea AF, Connolly DC, Huang L, Blancafort P. Targeting serous epithelial ovarian cancer with designer zinc finger transcription factors. J Biol Chem. (2012); 287:29873-86. [NCBI PubMed Entry]
