Targeted Drug Delivery, Imaging and Therapy
Associate Professor Juliana Hamzah

Targeted Drug Delivery, Imaging and Therapy
Associate Professor Juliana Hamzah
Profile
Associate Professor Juliana Hamzah is the Cancer Council WA Research Fellow, Head of Laboratory Targeted Drug Delivery, Imaging and Therapy at the Harry Perkins Institute of Medical Research. She has over 13 years of research experience in developing targeted delivery technology platforms for imaging and treating diseases including cancer and atherosclerosis. Following the completion of her PhD in 2005, Juliana did her first postdoctoral training at WA Institute of Medical Research (2006-2009) with Professor Ruth Ganss on developing therapies against cancer angiogenesis. She was then recruited to join the Program of Excellence in Nanotechnology at Sanford-Burnham Medical Research Institute, California, USA, under the prestigious American Heart Association Postdoctoral Fellowship (2009-2012). As a postdoctoral fellow in the laboratory of Professor Erkki Ruoslahti, she gained expertise in engineering theranostic agents. Juliana returned to Perth in 2013 as a NHMRC/National Heart Foundation R.D. Wright Biomedical Fellow and established her research team at Harry Perkins Institute of Medical Research.
Research Overview
Her current research focuses on developing strategies to specifically target diseases such as cancer and atherosclerosis for diagnostic imaging and local therapeutic interventions.
- Cancer and cardiovascular disease
- Inflammatory vasculature, macrophages and extracellular matrix
- Tissue homing peptides and targeted delivery
- In vivo imaging
- Targeted therapy
- Nanotechnology
Research Projects
A major challenge to detect and treat chronic inflammatory diseases such as cancer and atherosclerosis (i.e. hardening of the arteries due to fat accumulation) is to effectively deliver contrast agents and therapeutics into the pathological tissues whilst avoiding off-target binding and consequent cytotoxic effects. Our team focuses on developing tools and strategies to target the microenvironment of cancers (i.e. breast carcinoma, insulinoma and hepatocellular carcinoma) and atherosclerotic plaques for imaging and therapy. We have recently characterised a number of small molecules (i.e. peptides) that specifically bind to the abnormal cellular and non-cellular components in cancers and atherosclerotic lesions, including blood vessels, macrophages and extracellular matrix. These tumour and plaque –specific peptides can then be used as a drug delivery agent into the pathological tissues. Using chemical coupling and bioengineering approaches, we are now developing imaging contrast agents and therapeutics fused with these targeting peptides for in vivo applications in pre-clinical models of cancers and atherosclerosis.
Figure 1: Peptide homing in vivo in tumours and plaques. Source: Unpublished
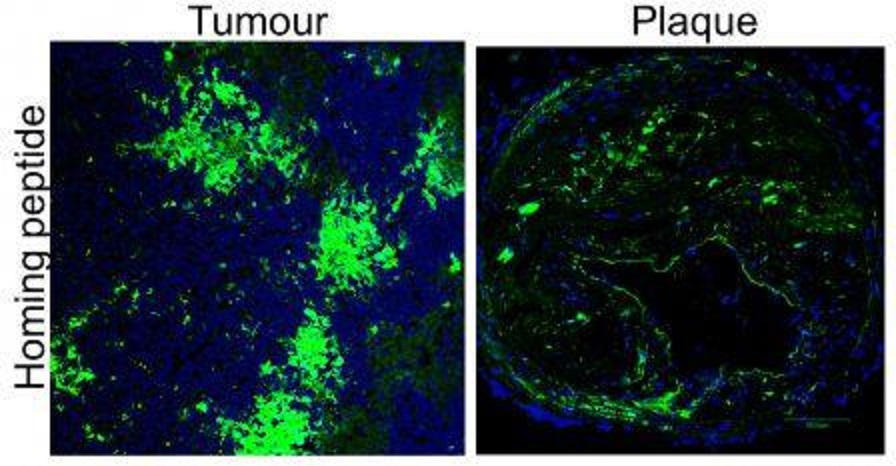
Improved diagnostic imaging for detecting cancers and atherosclerosis in vivo
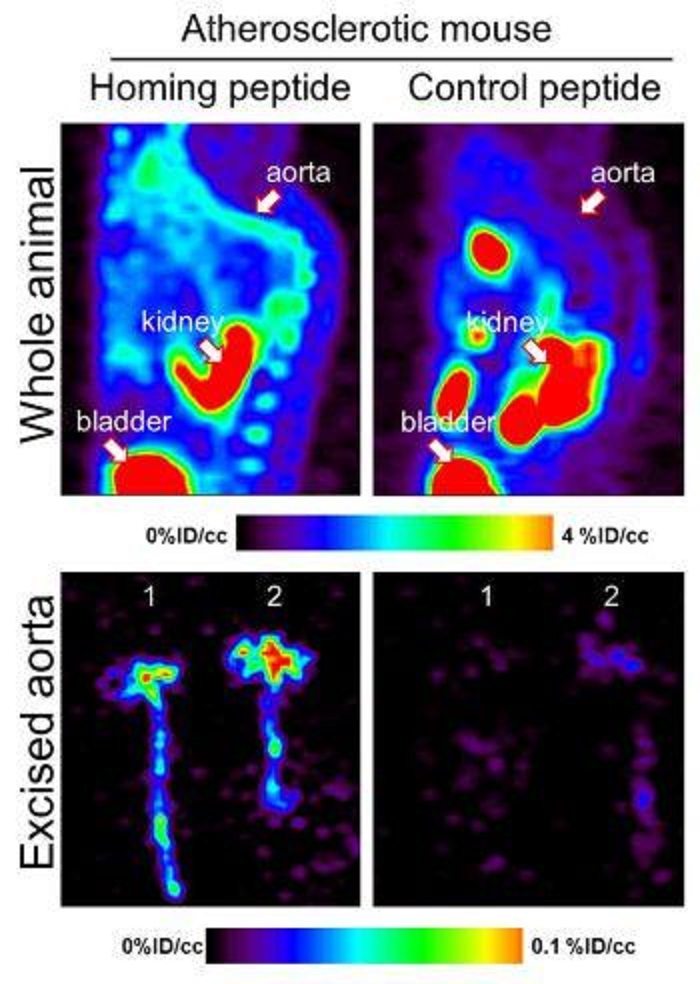
We have developed multifunctional nanoparticle-based system to carry contrast agents to image tumours and plaques using advanced imaging instrument including microPET/CT, MRI and confocal imaging. Importantly, these reagents are fused to the homing peptides to improve binding and accumulation deep in tumours and plaques.
Our goal is to be able to detect the developing tumours and atherosclerotic lesions at their earliest form as well as at the advanced chronic stages that are known to cause severe complications.
Figure 2: PET imaging of atherosclerosis in vivo. Source: Hamzah et al. PNAS, 2011
Targeted therapy to destroy pathological lesions and reverse inflammation in cancers and atherosclerosis
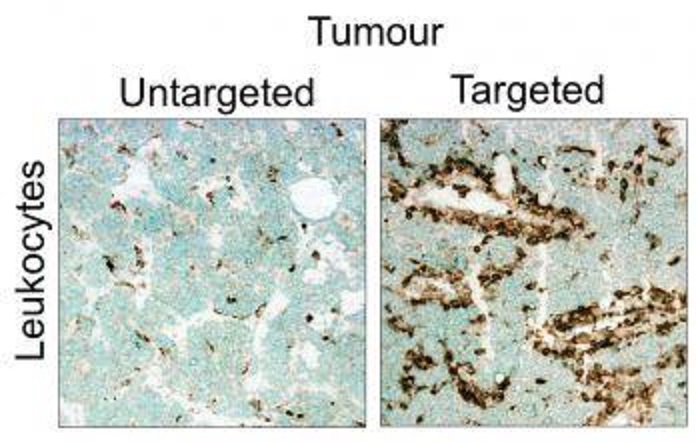
Our team explores two fundamentally different therapeutic interventions which involve targeting tumours and plaques to: i) destroy selective cellular components that contribute to the progression of cancers and atherosclerosis, and ii) re-program the diseased-promoting microenvironment by reversing specific inflammatory condition in the pathological tissues.
We engineered several classes of cell-killing agents and inflammatory mediators fused with tumour and plaque targeting peptides for effective delivery. We aim to monitor the drug penetration in tumours and plaques (by imaging) and evaluate the implications of these therapeutic strategies on cancer and plaque progression.
Figure 3: Immune cell infiltration in tumours in response to targeted delivery. Source: Unpublished
Student project opportunities
Please contact Juliana directly for project opportunities.
Selected Publications
Z. G. She*, J. Hamzah*, V. R. Kotamraju, H. B. Pang, S. Jansen, E. Ruoslahti, Plaque-penetrating peptide inhibits development of hypoxic atherosclerotic plaque. Journal of controlled release : official journal of the Controlled Release Society 238, 212-220 (2016); published online EpubSep 28 (10.1016/j.jconrel.2016.07.020). * Co-first authors.
A. Johansson, J. Hamzah, R. Ganss, More than a scaffold: Stromal modulation of tumor immunity. Biochimica et biophysica acta1865, 3-13 (2016); published online EpubJan (10.1016/j.bbcan.2015.06.001).
A. Johansson-Percival, Z. J. Li, D. D. Lakhiani, B. He, X. Wang, J. Hamzah, R. Ganss, Intratumoral LIGHT Restores Pericyte Contractile Properties and Vessel Integrity. Cell reports 13, 2687-2698 (2015); published online EpubDec 29 (10.1016/j.celrep.2015.12.004).
J. W. Seo, H. Baek, L. M. Mahakian, J. Kusunose, J. Hamzah, E. Ruoslahti, K. W. Ferrara, (64)Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjugate chemistry 25, 231-239 (2014); published online EpubFeb 19 (10.1021/bc400347s).
A. Johansson, J. Hamzah, R. Ganss, License for destruction: tumor-specific cytokine targeting. Trends in molecular medicine 20, 16-24 (2014); published online EpubJan (10.1016/j.molmed.2013.10.002).
L. Roth, L. Agemy, V. R. Kotamraju, G. Braun, T. Teesalu, K. N. Sugahara, J. Hamzah, E. Ruoslahti, Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 31, 3754-3763 (2012); published online EpubAug 16 (10.1038/onc.2011.537).
A. Johansson*, J. Hamzah*, C. J. Payne, R. Ganss, Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proceedings of the National Academy of Sciences of the United States of America 109, 7841-7846 (2012); published online EpubMay 15 (10.1073/pnas.1118296109). * Co-first authors.
A. Johansson, J. Hamzah, R. Ganss, Intratumoral TNFalpha improves immunotherapy. Oncoimmunology 1, 1395-1397 (2012); published online EpubNov 1 (10.4161/onci.20981).
O. Erster, J. M. Thomas, J. Hamzah, A. M. Jabaiah, J. A. Getz, T. D. Schoep, S. S. Hall, E. Ruoslahti, P. S. Daugherty, Site-specific targeting of antibody activity in vivo mediated by disease-associated proteases. Journal of controlled release : official journal of the Controlled Release Society 161, 804-812 (2012); published online EpubAug 10 (10.1016/j.jconrel.2012.05.035).
J. Hamzah, V. R. Kotamraju, J. W. Seo, L. Agemy, V. Fogal, L. M. Mahakian, D. Peters, L. Roth, M. K. Gagnon, K. W. Ferrara, E. Ruoslahti, Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 108, 7154-7159 (2011); published online EpubApr 26 (10.1073/pnas.1104540108).
