
Low dose colchicine gets FDA Approval for secondary prevention of heart disease on the back of Perth led clinical trial
Colchicine, a widely available gout medication, has received FDA approval to be used in low dose to prevent cardiovascular events in patients with proven coronary disease.
The approval to use low dose colchicine in this way comes following the discovery by Perth clinical cardiologist Dr Mark Nidorf (pictured above, left), and Professor Peter Thompson AM (above, right) from the Harry Perkins Institute of Medical Research. Their initial findings were published in the American Journal of Cardiology in 2007 and were followed by the ground-breaking LoDoCo (Low Dose Colchicine) trial in 2013.
“FDA approval is a strong affirmation by the most influential regulatory authority in the world that the results of our LoDoCo trials are valid and that low dose colchicine is both effective and safe to use in patients with coronary disease,” Professor Thompson said.
“This landmark work has established low dose colchicine alongside aspirin and statin therapy as a new corner stone treatment for coronary heart disease. Importantly colchicine is inexpensive and readily available as it has been repurposed for this indication. Up until now it has predominantly been used to treat gout.”
It is estimated that heart disease costs the Australian economy over $11.8 billion per year and is currently the leading cause of death in Australia despite improvements from cessation of smoking, better diet, better control of blood pressure and cholesterol.
FDA approval comes more than 10 years after the initial LoDoCo clinical trial was conducted with 500 patients in Western Australia between 2008 and 2012. Results from that trial showed that it had the potential to significantly reduce the risk of major cardiovascular events in patients with chronic heart disease. This result required further testing in a larger trial.
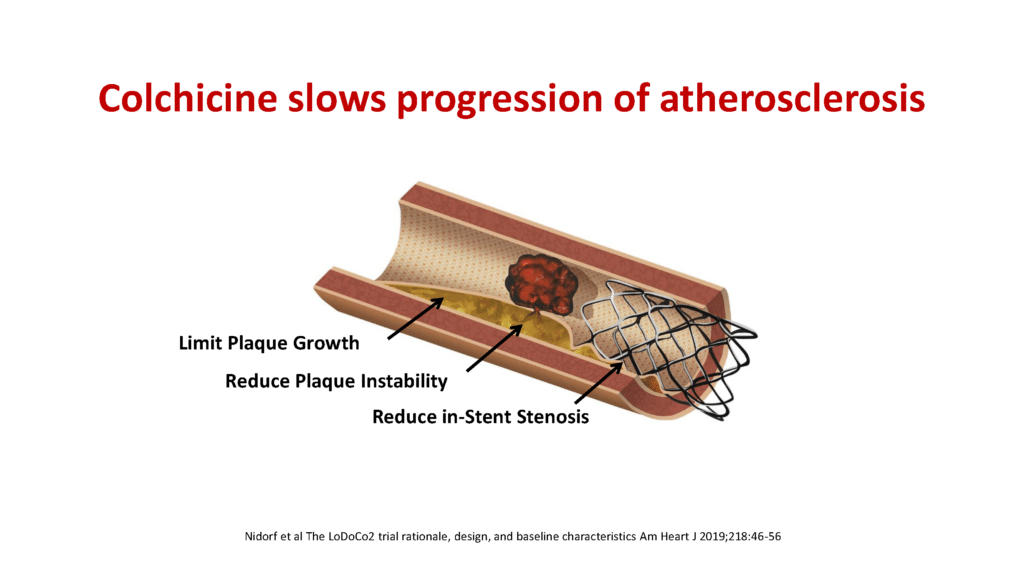
Dr Nidorf explained: “The dramatic results of our initial LoDoCo trial sparked global interest in this potentially game-changing treatment. We therefore designed and conducted a second large multicentre clinical trial in over 5000 patients. This trial was called LoDoCo2 and was an international collaboration between West Australian researchers and colleagues in The Netherlands. The commitment of the patients throughout the five-year trial was impressive, and there was a low rate of withdrawal from the trial and follow up was complete.”
The results of the LoDoCo2 trial, published in the New England Journal of Medicine in 2020, showed that there was a 31% reduction of major adverse cardiovascular events in patients on optimal medical therapy with cholesterol-lowering drugs.
Results of the LoDoCo2 trial also indicated that a low dose of 0.5mg of colchicine per day was well tolerated and appeared safe without any increase in the incidence of cancer or interaction with other commonly used medications including statin therapy.
Since LoDoCo2 was reported, over 11,000 patients in other clinical trials conducted in Australia and Canada have confirmed the benefits and safety of adding low dose colchicine to usual medical therapy in patients with coronary disease. In the next few years two other trials that collectively include over 10,000 patients will report the effect of colchicine in patients who have had a recent stroke or heart attack.
Dr Nidorf went on to explain how colchicine might work: “While colchicine has many actions, the most likely explanation for its effect is to dampen the inflammatory reaction which occurs when cholesterol changes from a relatively benign semi-liquid form to a highly irritant crystalline form within the atherosclerotic plaque. This is similar to the anti-inflammatory benefit the drug has in gout when uric acid changes to a crystalline form inside a joint, causing painful gouty arthritis.”
 Low dose colchicine is now registered for secondary prevention in patients with heart disease in the US, Canada, and South America and included in guideline therapy in Europe. Given the FDA decision, it is hoped that the Therapeutic Goods Administration (TGA) will also approve colchicine for this purpose in Australia in the near future.
Low dose colchicine is now registered for secondary prevention in patients with heart disease in the US, Canada, and South America and included in guideline therapy in Europe. Given the FDA decision, it is hoped that the Therapeutic Goods Administration (TGA) will also approve colchicine for this purpose in Australia in the near future.
ENDS
References:
- Nidorf S, Thompson P, Effect of Colchicine (0.5 mg Twice Daily) on High-Sensitivity C-Reactive Protein Independent of Aspirin and Atorvastatin in Patients With Stable Coronary Artery Disease, The American Journal of Cardiology, 2007 Mar, 99 (6) 805-807
- Nidorf S, Eikelboom J, Budgeon C, Thompson P, Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. Journal of the American College of Cardiology. 2013 Jan, 61 (4) 404–410.
- Nidorf S, Fiolet A, Mosterd A, … Thompson P, Colchicine in Patients with Chronic Coronary Disease, New England Journal of Medicine, 2020 Nov, 383 (19) 1838-1847
